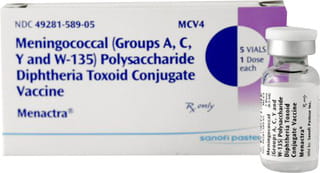
Menactra Vaccine
Description
Menactra vaccine is indicated for active immunisation against invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135 in individuals aged 9 months to 55 years. It does not prevent disease caused by N. meningitidis serogroup B. Menactra Vaccine is administered via intramuscular injection.
Some common side effects of this vaccine include reactions at the injection site, such as pain, swelling, or redness; irritability; loss of appetite; sleepiness; headache; nausea; vomiting; and diarrhoea. If these side effects do not improve over time or worsen, inform your doctor.
Inform your doctor about any other illnesses you may have had before receiving the vaccine. Disclose all medications you are currently taking. Pregnant and breastfeeding mothers should consult their doctor for advice before getting the vaccine.
Product Summary
| Offer Price | ₹4680.00 |
| You Save | ₹1020.00 (18% on MRP) |
| Contains | Diphtheria Toxoid(48.0 Mcg)+Meningococcal A, C, W And Y Conjugate Vaccine(16.0 Mcg) |
| Uses | Vaccine for meningococcal disease |
| Side effects | Pain at the injection site, headaches, fatigue, and injection site reactions. |
| Therapy | VACCINE |
Uses
Contraindications
- If you are allergic after a previous dose of a vaccine containing meningococcal capsular polysaccharide, diphtheria toxoid. If you are allergic to any component of Menactra Vaccine.
- If you have a moderate or severe fever or an acute illness, vaccination should be postponed until you have recovered.
Side effects
- Children Aged 9 to 12 Months: The most frequently reported reactions were tenderness at the injection site and irritability.
- Individuals Aged 2 to 10 Years: The most frequently reported reactions included pain at the injection site and irritability. Diarrhea, drowsiness, and anorexia were also common.
- Adolescents Aged 11 to 18 Years and Adults Aged 18 to 55 Years: The most commonly reported reactions after a single dose were pain at the injection site, headache, fatigue and injection site reactions....
- Booster Vaccination for Ages 15 to 55 Years: The most common reactions were pain at the injection site and muscle pain.
Precautions and Warnings
Pregnancy
Breast Feeding
Driving
Alcohol
Other General Warnings
- You have kidney disease or have had a kidney transplant.
- You have liver disease or a history of significant liver problems.
- You have ever been diagnosed with Guillain-Barré Syndrome (GBS), as this condition may recur after vaccination.
- You have a bleeding disorder or a low platelet count (thrombocytopenia), as the vaccine is administered intramuscularly and may pose a risk of bleeding.
- You are taking immunosuppressive medication or have a weakened immune system due to illness or treatment, the vaccine may not be as effective for you.
- You have a history of severe allergic reactions to any vaccine or its components.
- You tend to faint easily after injections (fainting or “syncope” has been reported after this vaccine). Precautions should be taken to avoid injury.
Directions for Use
- Menactra vaccine is administered as a single 0.5 mL dose via intramuscular injection, preferably in the anterolateral thigh for infants and young children and in the deltoid region for older individuals....
- For children aged 9 to 23 months, a 2-dose series is required, with doses administered at least three months apart. For individuals aged 2 to 55 years: A single dose is sufficient.
- Booster Vaccination (ages 15 to 55 years): A single booster dose may be given if at least 4 years have elapsed since the previous dose and the individual remains at ongoing risk for meningococcal disease....
Storage and disposal
Quick Tips
- Menactra vaccine is recommended for individuals between 9 months and 55 years of age.
- Menactra vaccine helps protect against meningococcal disease caused by serogroups A, C, Y, and W-135, but it does not protect against serogroup B.
- Menactra vaccine is given by intramuscular injection, typically in the thigh for infants and in the upper arm (deltoid muscle) for older children and adults.
- Common side effects include pain, redness, or swelling at the injection site, along with irritability, tiredness, headache, nausea, or diarrhea.
- Serious allergic reactions are rare, but you should seek immediate medical attention if you experience symptoms like swelling, hives, or difficulty breathing.
Dosage
Overdose
Missed a Dose
Mode of Action
How Does It Work?
Interactions
Interactions with other medicines
Content Details
Ravindra Ghongade
B. Pharm
Dr. Nikita Toshi
BDS (Bachelor of Dental Surgery), WHO FIDES member
Frequently Asked Questions (FAQs)
Q: Can Menactra vaccine cause side effects?
Q: Who should not receive the Menactra vaccine?
Q: Does Menactra vaccine protect against all types of meningitis?
Q: Can I receive the Menactra vaccine if I'm pregnant or breastfeeding?
Q: What should I do if I miss a dose?
Q: Can Menactra vaccine interact with other vaccines or medicines?
Q: What should I do if I experience a reaction after receiving the Menactra vaccine?
Q: Is Menactra vaccine a meningococcal vaccine?
References
- Menveo Group A,C, W135 and Y conjugate vaccine - Summary of Product Characteristics (SmPC) - (emc) [Internet]. www.medicines.org.uk. Cited [14th April 2015]
- Package Insert - Menactra | FDA [Internet]. Fda.gov. 2025 [cited 2025 Oct 28].
- Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine Menactra ® [Internet]. [cited 2025 Apr 14].
Did you find this medicine information helpful?
Please rate your experience