Could Favipiravir Be The Drug That Treats Covid-19?
By Dr. Nikita Toshi +2 more

Get,

to manage your symptom
Get your,


4 Cr+ families
benefitted

OTP sent to 9988776655



You’ve successfully subscribed to receive
doctor-approved tips on
Whatsapp

Get ready to feel your best.

Hi There,



Register to Avail the Offer
Send OTPBy continuing, you agree with our Privacy Policy and Terms and Conditions

Hi There,

Trusted by 4 crore+ families

OTP sent to 9988776655



You have unlocked 25% off on medicines




Code: NU25

By Dr. Nikita Toshi +2 more
The world had realized long ago that the only way to bring back normalcy to our lives & to the global economy is through vanquishing COVID-19. And the world-wide push to discover medication for this viral infection is going on full throttle.
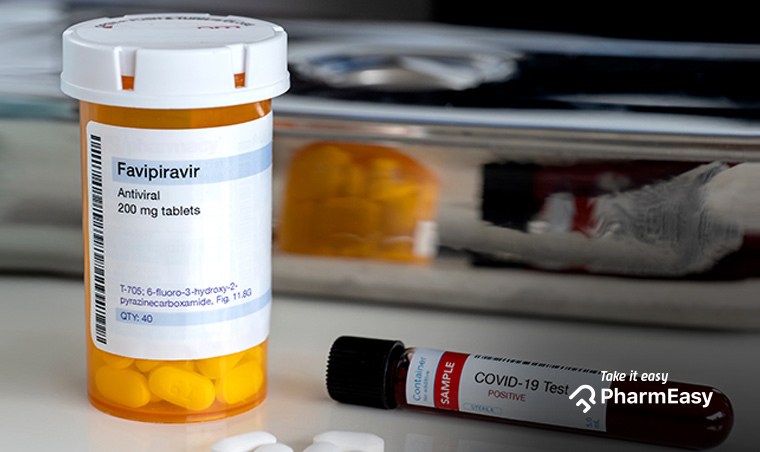
As of now, pharmaceutical companies are still trying to come up with a proven and approved drug to treat COVID-19. With the case count in India shooting up daily and the pandemic showing no sign of slowing down, Indian pharmaceuticals too have been racing against time to contain the damage. With more than 4.4 lakh people testing positive, the situation is starting to look grim. But there is a ray of light!
Table of Contents
The name of a medicine Favipiravir has been doing the rounds over the last few days. What is it and why has it got the scientific community buzzing?
Glenmark Pharmaceuticals, which has been foremost in the research for a COVID-19 drug has recently launched an anti-viral medicine Favipiravir, that is found to yield promising results. Glenmark Pharmaceuticals invented the active ingredient, API for this drug through its in-house research team. It announced in a press release that it had earned the DCGI’s approval to manufacture and market the drug under the brand name FabiFlu. It believes that in the grimly escalating situation that prevails in India, an orally administered drug will be more helpful.

Note that FabiFlu is not an established cure for COVID-19 as of today, clinical trials and research are still ongoing to come to a conclusion about the efficacy, the safety of the medicine. The clinical trials and reports published by Glenmark state that this new medicine has shown potential in treating mild to moderate cases of coronavirus.
What is the current status of FabiFlu?
For a medicine to be approved in India, it needs to go through robust clinical research in animals, followed by humans. The same data is then submitted to the government. In the case of FabiFlu, the clinical trials that establish safety and efficacy are still being conducted. As there is currently no established cure for COVID-19 and the number of cases rising, the government has approved Glenmark to conduct phase 3 trials and meanwhile manufacture and market the medicine.

This medicine’s safety is still being established in large clinical trials (phase 3). Hence its use is going to be very restricted and controlled for now. It will be prescription medicine only. Dosage shall be as prescribed by a registered medical practitioner based on the patient’s medical history and condition.
This medicine is only for emergency restricted use. It means before taking this medicine, each patient will be explained in detail the benefits and risks, the patient will be made to understand the safety, precautions and only when the patient is convinced, he will have to sign an Informed consent form. The same shall also be signed by the doctor as well. Once this is done, only then the patient shall receive FabiFlu. This should never be self-medicated as there are several safety concerns, precautions you need to follow even when taking this medicine and even a few days after discontinuing the treatment. Only when the same has been explained to you by your treating doctor, should this be taken.
Each tablet will be priced at INR 103.
The development of a new drug at a time of this immense crisis seems to offer us hope in the fight against COVID-19.
Disclaimer: The information provided herein is accurate, updated, and complete as per the best practices of the Company. Please note that this information shall not be treated as a substitute for physical medical consultation or advice of a registered medical practitioner. We do not guarantee the accuracy and completeness of the information so provided. The absence of any information and/or warning to any drug shall not be considered and assumed as an implied assurance of the Company. We do not take any responsibility for the consequences arising out of the aforementioned information and strongly recommend you for a physical consultation from a registered medical practitioner in case of any queries or doubts. Please click here for detailed T&C

Leave your comment...
Comments