Are you also one of those people who are worried over the shortage of COVID-19 vaccines forcing you to wait for the second dose? Are you also wondering if you may need to restart your vaccine schedule because it has already been months since your first dose?
Well, you do not need to panic yet as the studies and experts suggest that the booster (second) dose works just fine even if you take it after a gap of 4-5 months from the date of your first jab.
When you take the first dose of any COVID-19 vaccine, it triggers an immune response, which stays active even if you delay your booster dose. It, however, doesn’t work at the optimum level and that’s why you need the second dose.
The booster dose basically enhances and increases the immune response of the first dose-response.
Once the first dose of vaccine is taken, our body starts reacting and produces antibodies specific to the vaccine. The amount of time required by the body to produce an immune response may take time, but it triggers the memory cells of the immune system. When the second dose is given the immune response against the vaccine will be much faster and more effective than the first dose. Thus, a delay in the second dose will not harm and does not wear off.
Did you know?
Amid the rising demand and insufficient supply of vaccines, the government has increased the duration between two Covishield doses from 4 to 12 weeks. The decision has been taken after analyzing the data from the international trials, in which the researchers observed the immune response of the vaccinated people from the first dose till the booster jab.
The experts also observed that in some cases where the second dose was administered as late as 12 weeks, the antibodies and immune response from the vaccine were equivalent and comparable.
Some experts believe that from an immunology perspective, researchers always specify a ‘minimum’ interval, not ‘maximum’ between the doses. They believe that any vaccine works better if the gap between the first and the booster dose is slightly longer in most cases.
In the case of Covaxin, the minimum gap of 4-6 weeks remains unchanged. In case the vaccine is not available, it can be delayed but has been mentioned for 28 days but everyone must get their second dose of the COVID-19 vaccine even if it is delayed.
Some experts also recommend that people should not think about taking the first dose again if the booster jab has been delayed. Even in children’s vaccination, the norm is to pick up from where the one left off.
Moreover, even children are not recommended a repeat dose of the same vaccine even if they have a weaker immune response. Hence, it should not be done for the COVID vaccination too.
Another expert has claimed that the second dose of the COVID-19 vaccine, even if delayed, is effective in preventing the infection.
There have been cases where people contracted covid within a few days or weeks of getting their first dose of COVID vaccination. In case a person contracts the infection very close to getting their second COVID-19 dose, here’s what you should do. Remember that the infection helps the body build certain protective antibodies naturally, but we don’t know for sure how long these antibodies last and hence it is advisable to get the second dose of the vaccine after recovering.
You must postpone your vaccination by at least 6 weeks after a mild covid infection. Those with a severe form of illness should consider getting a go-ahead from the doctor first. Make sure you recover well but do not miss the vaccine dose as it will add to your immunity and serve additional purposes.
In the United Kingdom, the recommended gap between the two shots of Covishield is 12 weeks, while Canada has kept it to 16 weeks. A study by Public Health England has proved that the first jab of Covishield is 65 percent effective against symptomatic COVID-19 and 80% against a severe form of the disease.
Some experts even recommend that even if a person gets infected after getting the first dose of the vaccine and takes 6-10 weeks to recover, he/she must take the second jab after 3 months of being completely recovered from the illness.
First of all, you should not worry at all about the revision of the vaccination schedule. The decision has been taken after a thorough analysis of every aspect of the efficacy of the doses. Still, if you are unable to get the vaccine at a government-designated centre, you can always look for a private vaccination centre in your locality and get the vaccine.
The shortage of vaccines may have sparked a wave of fear among the people who have got their first shot of the vaccine, but there isn’t anything to worry about as of now. There would be no need to restart the vaccine schedule even if you have completed 12 weeks from your first dose. Everyone should just focus on getting their second dose as per the government-specified schedule.
Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
As India is grappling with the Coronavirus pandemic, people are running from pillar to post to arrange hospitals, ICU beds, life-saving medications, ventilators and most importantly ‘oxygen’, to save their loved ones.
Arranging these basic necessities is so tough today because the availability of all these things is way less than what the country requires to win this battle.
In these testing times, Oxygen Concentrators have turned out to be a ‘saviour’ for the people who are mildly affected with COVID-19 and are trying to recover in home isolation, owing to the shortage of hospital beds.
An oxygen concentrator is a medical device that is generally bigger than a computer monitor. It works by concentrating oxygen from the ambient air and aiding the patient in breathing easily.
In today’s polluted world, atmospheric air contains about 78 percent nitrogen and 21 percent oxygen. The last one percent consists of various other gases.
An oxygen concentrator sucks this atmospheric air, filters it through a sieve/filter and releases the nitrogen back into the atmosphere. The filtered oxygen is given to the patient through a cannula.
The studies have shown that these concentrators produce 90-95 percent pure oxygen. The World Health Organisation (WHO) in 2015 stated, ”Concentrators can operate and produce oxygen 24 hours a day continuously and may last up to five years.”
As the majority of the states in India are facing scarcity of oxygen right now, people should start taking the help of concentrators when their oxygen saturation level drops below 94 percent. Notably, oxygen saturation level between 94-100 percent is considered safe and healthy.
These concentrators are effective on patients whose saturation level doesn’t drop below 90 percent, but they can be of help even for those with oxygen saturation dropping as low as 85 percent. Patients, whose oxygen level drops below this point, will require a better flow of oxygen than these concentrators can offer.
The experts claim that oxygen concentrators are not as good as Liquid Medical Oxygen (LMO), which are 99 percent pure and are a good option for mild to moderate COVID-19 patients, whose oxygen saturation level is at 90 percent or above.
The experts say that these are not appropriate for ICU patients.
Basically, there are two types of concentrators – Continuous flow and Pulse dose.
Continuous flow concentrators keep supplying the same flow of oxygen every minute if they are not turned off. The pulse dose concentrators are a little smarter and read the breathing pattern of the patient and release O2 on detecting inhalation. In the second type, the per-minute oxygen dispensation will vary.
These are the best alternatives of the cylinders and LMO, which are very tough to be transported and stored. Moreover, cylinders require to be refilled but concentrators can keep producing oxygen for up to five years or more, using just the ambient air and a power source.
The only drawback with the concentrators is that they can only supply 5-10 litres of oxygen per minute, while the critical patients may require 40-45 litres per minute.
The cost of these concentrators varies depending upon how much oxygen they produce per minute. Their cost may vary between Rs. 40,000 and Rs. 90,000 in different markets.
As per the experts, if a patient is given one-litre oxygen through a concentrator, the oxygen percentage in the lungs shoots up to 24 percent, while with two litres, it reaches 28 percent and with 10 litres, it rises up to 60 percent. The oxygen per minute has to be regulated as per the requirement.
The patient must consult a physician to know how many litres per minute of oxygen is needed and a pulse oximeter is also necessary to monitor the oxygen saturation.
The quality of oxygen produced depends upon the number of sieves/filters used in the concentrator and their quality is not measured by their weight but by litre per minute output. The ideal weight of these concentrators is less than 27 kgs.
If the concentrator gets heated while working, the quality of oxygen produced may deplete. Hence, it should be used only when required.
The coronavirus pandemic has given a huge boost to the oxygen concentrator sales in India, as their demand has shot up shockingly. Earlier, where the demand was 40,000 concentrators annually, now it has gone up to 30,000-40,000 a month.
Well, oxygen concentrators have been an essential part of every home in China where there are people over 65 years of age. With COVID-19 infecting an increasing number of people on a daily basis, these concentrators can really help the Indian healthcare system.
Grab a concentrator and book a test for advanced health checkup.

Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
The new COVID-19 strain has derailed the Indian healthcare infrastructure in no time, and our country, which was donating vaccines to the neighbouring nations a few weeks ago, is facing a shortage of the life-saving shots and almost every other facility required to fight this pandemic.
In a huge relief to the people, the Drug Controller General of India (DCGI) has approved the Sputnik V COVID-19 vaccine for emergency use in India. This is the third vaccine India has approved to use against the deadly virus. The earlier two are – Covishield and Covaxin. The former has been developed by the Oxford University-AstraZeneca and Pune-based Serum Institute of India (SII) is manufacturing it, while the latter has been developed and manufactured by Bharat Biotech and the National Institute of Virology (NIV).
Indian pharmaceutical company Dr Reddy’s Laboratories has joined hands with the Russian sovereign fund Russian Direct Investment Fund (RDIF) for carrying out the bridge clinical trials of the vaccine in India, which is the 60th country to approve its use.
The RDIF has confirmed that vaccine distribution will begin by the end of April or in the first week of May.
The Gamaleya Research Institute of Epidemiology and Microbiology, backed by the Russian state, has developed the vaccine. The registration of the vaccine in Russia was done in August 2020 as Gam-COVID-Vac, and the “V” in the name of the vaccine stands for alphabet V. The developer has informed that the vaccine can be stored at 2-8 degree celsius.
Dr Reddy’s Laboratories has got the contract of distributing up to 250 million doses in India, by importing them. RDIF has also signed a manufacturing contract with Stelis Biopharma, Gland Pharma, Virchow Biotech, Panacea Biotec and Hetero Biopharma to manufacture more than 850 million Sputnik V doses in India. The doses produced in India will be exported across the world.
The production of the vaccine is soon to start at Hetero Biopharma and Panacea Biotec, while the other pharma companies will begin production in September 2021.
Sputnik V is an adenovirus viral vector vaccine, which carries viruses especially made containing the coronavirus genes. Some of these types of vaccines make their way into the cells and cause them to produce viral proteins. The others just gradually replicate, travelling through the body with virus proteins on their surface.
People have to get two doses of Sputnik V, similar to Covishield and Covaxin. Both the doses have to be administered at least 21 days apart. These are inoculated through injection into the muscle.
The Russian vaccine has shown excellent results with an efficacy rate of 91.5 percent. This rate is the highest after Moderna and Pfizer-BioNTech’s vaccines. Before giving it the green signal, a bridging trial was conducted in India.
Another positive news is that only 0.1 percent of the vaccine receivers have complained about the side effects.
In the international market, the Russian vaccine costs around Rs. 750 per dose, but its price in India is still to be decided, as the government is trying to negotiate the pricing with the manufacturer.
The vaccine approval for emergency use in India is very crucial as the second wave of the pandemic has already put India on the back foot in the war against the virus. Many states in the country have been reporting a shortage of vaccines, even when India is all set to expand the vaccination drive.
Russia became the first country to start inoculating its citizens with Sputnik V. It is currently being administered in many countries including Pakistan, Egypt, Argentina, Vietnam, Morocco, Bahrain, Jordan, Panama, Mauritius, Hungary, the United Arab Emirates, Iran, the Philippines and Sri Lanka.
With the quickly rising number of cases of COVID-19 in India, approval to Sputnik V for emergency use can come as a boon for the countrymen. We should hope that the first lot of vaccines land in India soon so that the vaccination drive can come back on the right track.
Know your current health status.

Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
Right when India was starting to smell victory against the deadly COVID-19 disease, the virus has struck back with a new strain, which in a way has derailed the ambitious vaccination drive of the Indian government.
The virus has restarted spreading its wings across India The World Health Organisation (WHO) has already confirmed that the new strain of the coronavirus is even more contagious than the previous one and at the same time, people in India have started to take it less seriously.
To investigate and figure out ways to stop the second wave of COVID-19, the central government formed a panel, which in its report, said, ”The main reason behind the second wave is the superspreader events like weddings, elections and social gatherings and people being unwilling to keep following the social distancing norms.
Niti Ayog member (Health) Dr VK Paul has suggested not to show any laxity in our fight against COVID-19, as breaking the chain of transmission should be our utmost priority at the moment. He also emphasized that vaccines are a major tool in our mission but we can not afford to give up on the containment and surveillance methods that we have been following for so long.
In the last 24 hours ending at 8 AM Monday, India recorded the highest ever 1,03,558 new COVID-19 cases. These cases have taken India’s total to 1,25,89,067, out of which 9.07 lakh are currently active, while 1.17 crore has recovered successfully. In the last 24 hours, a total of 478 deaths have been reported, taking the toll up to 1.65 lakh.
Maharashtra is the worst affected state right now with over 3.36 lakh active cases. The state recorded 31,643 cases on Monday itself. To curb the resurgence of the virus, the state is likely to release new work norms by April 1. The decision on a full lockdown will be taken in a meeting headed by the Chief Minister of the state.
In a strange occurrence, eight states of the country accounted for 84.5 per cent of the total 68,020 fresh cases recorded on Monday. Delhi also recorded more than 1,900 cases on Monday, breaking its past three-month record.
India’s indigenous vaccines – Covaxin and Covishield – have been performing well, but looking at the huge population of our country, it is impossible to immunize everyone in a short period. Till now, India has managed to vaccinate only six crore people, which is just 4.43 per cent of the 135.5 crore population of the country.
More shockingly, only 0.5 per cent of people in India had received both doses of vaccines by March 18, 2021. If Indian authorities maintain the same pace of vaccination, they would be able to vaccinate 70% of the population in the next 10.8 years.
Hence, it becomes essential for the people of the country to maintain the social distancing norms specified by the authorities, so that the government doesn’t have to use their time, manpower and focus on stopping the spread of the virus, instead of speeding up the inoculation drive.
Any government cannot succeed against the deadly virus if the people of the country do not contribute their bit to the mission. Hence, getting vaccinated timely should be our top priority and until we are not inoculated, we must follow the social distancing norms.
To stop the resurgence of COVID-19 in India, people must ensure that they are following all the protocols set by their local authorities, and also step forward to get themselves immunized against the disease. We must stop the spread of misinformation and myths related to the disease and vaccines. Moreover, people should also ignore attending social gatherings to stay safe.
Know your current health status!

Disclaimer: The information included at this site is for educational purposes only and is not intended to be a substitute for medical treatment by a healthcare professional. Because of unique individual needs, the reader should consult their physician to determine the appropriateness of the information for the reader’s situation.
Amid the advancement of COVID-19 vaccination drives across the globe, the confusion and rumours around the efficacy of the vaccines are still rising. People are trying to find answers to their questions like – ”When do they become immune to the virus?”, ”How long does the immunity last?” and many more.
Well, these confusions exist because people tend to believe every rumour they come across, while the World Health Organisation (WHO) has appealed multiple times to only trust the authentic sources about the information related to Coronavirus.
One of the most prevalent confusions around the Coronavirus vaccines these days is how long should a person wait to get the vaccine after getting infected.
Some of the researchers have recently claimed that a person can take the COVID-19 vaccine right after recovering from the disease but only if they do not have any symptoms. The Centers for Disease Control and Prevention (CDC), however, suggests waiting for 90 days from the day you test positive for COVID-19 disease if you haven’t received any shot yet.
The CDC also suggests, people who got infected after receiving their first shot may take the second dose on their scheduled date, but only if their quarantine period is over and they do not have any symptoms.
The CDC has also clarified that if you have had COVID-19 and now you have recovered, you must be having some natural protection against the virus, but it is still unclear how long that immunity is going to last.
COVID-19 vaccination helps protect you by creating an antibody response without you having to experience potentially severe illness or post-COVID conditions. Vaccination can be taken safely post 90 days of successful recovery from COVID.
Dr. M.G. Kartheeka, MBBS, MD
The CDC has suggested not to take the vaccine for at least 90 days if you have undergone an antibody therapy for COVID-19.
Currently, India has vaccinated over 62.5 lakh healthcare workers and frontline workers. The authorities have confirmed that about 3.3 lakh people are being inoculated on a daily basis.
The authorities, however, have cautioned COVID-infected people not to get the vaccine if they have any symptoms of the disease. Moreover, long-haulers, people who have recovered from COVID-19 but still have some symptoms, should consult their physician before taking the shot.
There are plenty of myths that are discouraging people from showing up at the vaccination centres, causing a huge blow to India’s fight against the Coronavirus. Here we have listed a few myths which CDC has described as completely baseless:
Many people around the country believe that those who are taking the vaccine shots are suffering from serious side effects. They agree that the people may suffer from some mild side effects, which are listed below:
More serious side-effects include:
With the kind of results India’s indigenous vaccines are fetching, people have started showing more interest in taking the shot for COVID-19. We, however, have to work together to stop the spread of misconceptions to eventually defeat the disease which had literally confined the world to their houses.
Let’s pledge not to forward any information related to the disease without cross-checking it with the official sources, like the Ministry of Health and Family Welfare, WHO, CDC, etc.
Know your current health status.

Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
As India has finally successfully developed its own vaccine to overcome the ongoing COVID-19 pandemic, everyone is wondering when they would get a shot of it, which most probably may restore the actual “normalcy” in their life.
Well, an expert panel with the government’s health ministry, the National Expert Group on Vaccine Administration (NEGVAC), has come up with a prioritisation list to identify the most vulnerable populations in an effort to reduce the mortality rate and burden on the healthcare system.
As every one of you was already guessing, healthcare workers (HCWs) are the first in line to get the vaccine. It means, doctors, nurses, every worker in the healthcare setup, will be the first to get the vaccines. As per the NEGVAC, there are about 1 crore HCWs across the country.
The second slot in the priority list is reserved for frontline workers (FLWs), who include state and central police department cops, home guard, armed forces and civil defence organizations, including municipal workers and disaster management volunteers. The overall population of FLWs in the country is about two crores.
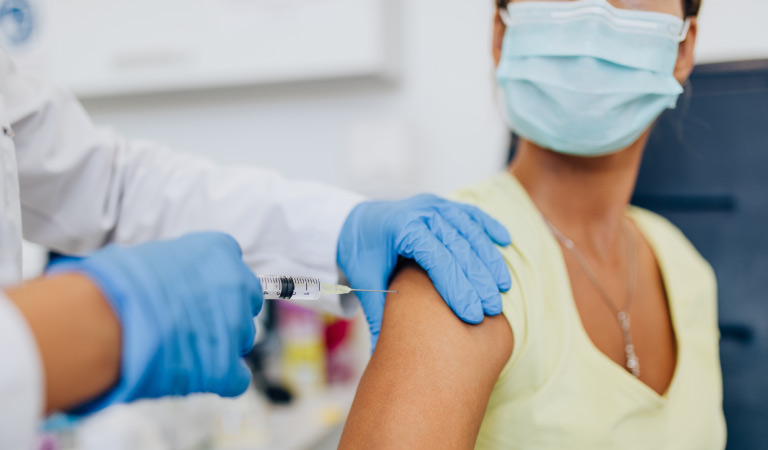
After covering these two groups, the expert panel has finalized a prioritized age group, which covers people above 50 years of age, and those below 50 years of age having associated comorbidities. As per the data collected by the expert panel, the population of such people is about 27 crore in India.
The age group of above 50 years has been subdivided into two parts – first of those above 60 years, and then of the people between 50 and 60 years. This bifurcation has been done for purposes of the phasing of roll out based on pandemic situation and vaccine availability.
Once the priority groups are covered, the remaining population will be vaccinated based on the disease epidemiology and vaccine availability.
The expert panel is referring to the latest electoral roll of Lok Sabha and Assembly elections to figure out the eligible candidates for the vaccinations. The age will be calculated as of January 1, 2021. So, those who were born on or before January 1, 1971, will fall under the category to be vaccinated as per the priority list.
Moreover, the government has already started collecting data on healthcare workers across the states, union territories, and central ministries. The collected data is being uploaded on CO-WIN software, a digital platform for real-time monitoring of COVID-19 vaccine delivery and it will be verified at multiple levels before beginning the vaccination process.
The beneficiaries are required to register on the CO-WIN software beforehand in order to avail the vaccine. The government hasn’t set any provision for on-the-spot registration for any candidate.
The central government of India has confirmed that they currently have 2.39 lakh vaccinators (Auxiliary Nurse Midwife-ANM), of whom only 1.54 lakh will be used to administer the COVID-19 vaccine shots. The aim of not using all of them is to minimize the effect on routine healthcare services across the country, including routine immunization.
The government has claimed that the current cold chain has the capacity of storing vaccines for the first three crore health workers across the country.
Over and above this, the government also confirmed that the plan and infrastructure are ready to kick start massive production of COVID-19 vaccines as soon as the scientists and researchers give a green signal.
Three major firms i.e. Serum Institute of India, Pfizer Inc and Bharat Biotech have applied for Emergency Use Approval in the last few days. The regulatory framework of the country has a specific provision for granting the emergency use authorization. Emergency use approval of the COVID-19 vaccines will be granted after sufficient evidence regarding their safety and effectiveness.
The NEGVAC has prepared a detailed and authentic plan for rolling out the COVID-19 vaccine, and the people, who do not fall in the selected categories for phase 1 rollout, should not panic. The panel surely has a plan in place for covering the remaining population of the country in the months to come.
Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
Moderna’s mRNA-1273 vaccine is co-developed by a biotechnology company – Moderna, Inc (Cambridge, Massachusetts.US) & the National Institute of Allergy and Infectious Diseases (NIAID).
It works by incorporating coronavirus’s genetic code into the human body. This leads to the generation of viral proteins, but not the whole virus, which is enough to train the immune system to attack. This would teach the body to make COVID-19 antibodies and develop another part of the immune system called T-cells to fight the coronavirus. This is one of the novel techniques of inserting RNA into human cells.
Moderna conducted a clinical trial involving 30,000 people in the US with half being given two doses of the coronavirus vaccine in a four-week gap & the rest had dummy injections. The analysis was based on the result of the first 95 volunteers to develop COVID-19 symptoms.
What was observed, was only five people tested positive for COVID-19 had been given the covid vaccine. The rest 90 were in those given the dummy treatment. The company thus concluded that the coronavirus vaccine 94.5% effective.
It also gave news of relief by confirming that there are no significant safety concerns. The basic side effects after administration of the covid 19 vaccine included short-lived fatigue, headache and pain.
Researchers are still studying the longevity of immunity for the volunteers who were administered the vaccine.
The preliminary data show very similar protection data which is around 90% for Pfizer/BioNTech vaccine and around 95% for Moderna vaccine. Since both the vaccines have the same mechanism of injecting part of the virus’s genetic code in order to provoke an immune response, we will have to wait for the final numbers in terms of safety & efficacy.
However, logistically Moderna’s coronavirus vaccine appears to be easier to store as it remains stable at minus 20’C for up to six months and can be kept in a standard fridge for up to a month. On the contrary, Pfizer’s covid-19 vaccine needs ultra-cold storage at around minus 75’C & it needs to be kept in the fridge for five days before administration. This gives an additional operational edge to Moderna over the Pfizer vaccine.
The Indian government is in negotiation terms with both domestic and international vaccine makers such as Moderna, Pfizer, Serum Institute, Bharat Biotech and Zydus Cadila for their Covid-19 vaccine.
Few Indian institutions such as Hyderabad-based Indian Immunologicals (IIL) are all geared up for a manufacturing collaboration with Moderna. The Indian government has also kept this possibility open with Moderna. They have also requested for direct purchase of the coronavirus vaccine and later internal distribution.
Global demand for covid 19 vaccines is expected to far exceed supplies despite significant efforts to increase the production ahead of time. Moderna has already signed a deal to supply 100 million doses to the U.S. and 80 million to the European Union. Other countries such as U.K & India are still in the negotiating phase.
This news from Moderna is very exciting & boosts optimism among people. This coronavirus vaccine news has definitely brought back hope in people across the world. But as responsible citizens, let’s aim to follow other precautionary measures till this vaccine finally comes to the market.
The COVID-19 outbreak has shaken the global health system and economy by its roots. This epidemic is continuously spreading and showing no signs of slowing down. Vaccination could be the only effective and economical means to control or stop this pandemic. Many research institutions and pharmaceutical companies worldwide are currently involved in the development of a suitable coronavirus vaccine.
The development of coronavirus vaccines consists of the following steps e.g. exploratory, pre-clinical and clinical stages.
1. Exploratory stage – It is the basic research in the laboratory of the conceptual idea and development of an antigen against the disease against which a vaccine needs to be produced which usually takes 2–4 years’ timeframe.
2. Pre-clinical stage of development – It uses a platform of tissue-culture and animal testing to assess the safety of the vaccine. They may also suggest the safest starting dose for the next phase of research as well as the safest method of administering the covid vaccine. This stage usually takes 1–2 years and out of 100 potential candidates, 6 usually pass through this stage.
3. Clinical stages of development – Consist of at least 3 stages and the 4th post-marketing safety assessment is also mandatory. These are performed on Human volunteers.

The efforts on coronavirus vaccine began initially in China as soon as the outbreak of coronavirus erupted and then world-over as the disease was declared a pandemic by WHO. Eventually, each country got into the race of developing the vaccine to be 1st in the world to safeguard its population & have an advantage over other countries.
Conclusion:
Covid 19 pandemic is raging, even escalating. That puts pressure on all the countries to rush for a covid vaccine. Under such pressure, countries may authorize emergency use of a vaccine on specified groups without waiting for the completion of Phase III trials which could be harmful. We all have shown great patience till now, let’s continue the same by taking all the safety measures & following Government guidelines.
COVID-19 vaccine drive –
More than 80 million people in India have received at least one dose of a coronavirus vaccine and 11.1 million people have been fully vaccinated in what is the world’s biggest inoculation drive. Here’s how you can book a COVID-19 vaccine for your loved ones.
From March 1 onwards, all people above the age of 60 will be eligible to receive the COVID-19 vaccine. This is the second phase of India’s COVID vaccination programme and it will benefit 10 crore people across the whole nation.
| Vaccine | Developed By | Country | Stage | Update |
| ChAdOx1-S | Oxford University and has been licensed to AstraZeneca | The United Kingdom and India | Phase 3 | South Africa has halted use of the AstraZeneca-Oxford coronavirus vaccine after evidence emerged that the vaccine did not protect clinical-trial participants from mild or moderate illness caused by the more contagious virus variant that was first seen in the country. |
| BNT162b2 Vaccine | Pfizer in collaboration with BioNTech & Fosun Pharmaceuticals | USA, Germany & China | Concluded Phase 3
U.K regulator on Dec 2 declared the vaccine for use. | FDA have described changes to the guidelines for the transportation and storage of the Pfizer-BioNTech vaccine. They explain that it can now be transported and stored at conventional temperatures commonly found in pharmaceutical freezers for a period of up to 2 weeks. If the FDA grants Pfizer’s request, the change could significantly speed up the vaccine rollout in the United States. |
| EpiVacCorona | Vektor Centre, Novosibirsk | Russia | Given regulatory approval | A clinical trial with 40,000 volunteers to begin soon |
| mRNA-1273 Vaccine | Moderna along with NIH | United States | Phase 3 | vaccine shows ‘no specific safety concerns. Results of the first analysis to be published by end of November. Early data shows 95% efficacy. |
| Sputnik V Vaccine | Gamaleya National Research Centre of Epidemiology and Microbiology | Russia | Developed.
Inadequate reports to prove the Vaccine’s efficacy and safety. | Phase 3 results analysis shows 92% efficacy. Phase 2 & 3 Trials in India to begin soon. Approved in Russia. |
| Covaxin | Bharat Biotech | India | Rollout begun | The most reliable longer-term protection against coronavirus is provided through vaccination, experts said noting that the immunity afforded by the presence of antibodies might be expected to last only several months. |
| ZyCoV-D | Zydus Cadila | India | Phase 1 & 2 | Has also begun working on a COVID drug to treat patients |
| CoronaVac Vaccine | Sinovac Life Sciences Co. Ltd. (China) in collaboration with Institution Butantan | China & Brazil | Phase 3 | Trials halted in Brazil. Turkey and Indonesia begin late-phase human trials |
| Ad5-nCoV
Vaccine | CanSino Biologics company in collaboration with Beijing Institute of Biotechnology | China | Phase 3 | Russia & Pakistan to begin Phase 3 trials |
| NVX-CoV2373 | Novavax | US | Phase 3 | Signed an agreement with SII to provide vaccine to low and middle-income countries post-approval |
| Janssen COVID-19 vaccine | Johnson & Johnson | US | Phase 3 | Preparations underway to roll out 1 billion doses in 2021 |
| Sanofi COVID-19 vaccine | Sanofi Pasteur in partnership with Translate Bio & GlaxoSmithKline | US & UK | Phases 1 & 2 | Phase 3 trials to commence by end of December |
Countries all over the world have been fighting against the novel coronavirus using preventive measures such as social distancing and lockdowns. After India being in lockdown for almost half a year, certain relaxations have gradually been implemented to resume economic activities.
Amidst the rising number of new cases daily, the first documented case of reinfection with Covid-19 was reported in a 33-year-old man in Hong Kong almost 4 ½ months after he recovered from his first infection. Closer to home, recently there have been reports of reinfection cases in Bangalore, Delhi and Mumbai, especially amongst healthcare workers. Researchers in India have also reported few cases with asymptomatic reinfection of Covid-19.
In simple words, reinfection means that a person was infected once, cleared the infection and then the person was infected again.
Initial evidence suggests that normally, in case of an infection, the COVID Immunoglobulin G (IgG) antibody is tested positive after 2-3 weeks of the infection. However, in certain cases, the antibody tests negative, which means that the person does not develop immunity after infection. Another possibility could be that the antibodies are short-lived and disappear quickly, thereby making the patient susceptible to reinfection. Thus, reinfection cases mean that the antibodies may not be produced by every patient or if they do develop, they may not last long enough, and therefore, allowing the virus to enter the body and cause the disease again.

The World Health Organisation (WHO) has stated that there is currently no evidence that people, who have recovered from COVID-19 and have antibodies, are protected from a second infection.
Though the patient from Hong Kong experienced a cough, sore throat, fever and headache during his first infection, he was asymptomatic during his second infection. On the contrary, a 25-year-old man in the US developed breathing difficulties and pneumonia after getting re-infected with COVID-19, despite having only mild symptoms during his first infection.
Although there could be a chance that the virus was quite low enough to go undetected and appear negative on a test, only to increase and be picked up in the next test, the question arises as to whether the same virus has resurfaced again or is the current infection caused due to another viral event.
Determining reinfection is quite a challenging process. Genetic material gathered from the first and second swabs of the patient must be analyzed and sequenced by using genetic engineering. If there are differences that show up during sequencing, it means that reinfection has occurred. If the sequences match, then it means that there could be a relapse.
As of now, health officials in India are of the opinion that reinfection with COVID-19 is very rare. Observations till date have shown that only mild infections have occurred and therefore, this issue is not of serious concern now.
As per early-stage reports from vaccine clinical trials being conducted worldwide, the vaccine produces less immunity than an infection. There is still a lot of testing and data that is required in order to ascertain the level of immunity that the vaccine would provide. However, we may require booster doses in order to maintain immunity and avoid infection.

Read more about: Difference between Covaxin vs Covishield Vaccine
Herd immunity is based on the principle that a disease can be eradicated if most of the population is immune to it. With cases of reinfection being reported and confirmed, researchers are increasingly sceptical about achieving herd immunity since it may be impossible to achieve. Once again, more research is needed in order to confirm this fact.
Recovery from COVID-19 does not mean that you are immune to the disease. You should avoid getting complacent and continue maintaining hand hygiene, wearing masks and ensuring social distancing even after recovery.
Since COVID-19 is a relatively new disease, there is not much data available to understand the kind of long-term immunity that patients develop after they have recovered from the infection.
With respect to the novel coronavirus and COVID-19, a lot is still left to be learned. Research is still underway in order to understand various aspects of the disease and its threat of reinfection as well as the vaccine and immunity. In all cases, health officials have advised that the chances of reinfection are rare and not a cause of serious concern.
Is it right to take care of your health first? Yes! It is.

Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.
As COVID-19 pandemic continues to wreak havoc worldwide, various data sources suggest that in India the recovery rate of COVID-19 is over 70% as of August 23, 2020. India currently has almost 2.4 million patients who have recovered from COVID-19. However, an important question still looms large – Does a COVID-19 survivor recover completely after hospital discharge?
The SARS-CoV-2 virus primarily affects the respiratory system and can cause life-threatening pneumonia. Current research shows the disease attacks more than just the respiratory system, affecting multiple organs with blood clots and inflammation. Almost 80% of COVID-19 infections are mild or asymptomatic, 15% are severe infections requiring oxygen and 5% are critical infections, requiring ventilation.
Several COVID-19 recovered patients are returning to doctors with conditions including breathlessness, cardiac, lung and other complications. According to a newly published study from Italy, many patients with even milder forms of COVID-19 have persistent symptoms of fatigue and difficulty breathing for up to 60 days post-infection. Furthermore, researchers of this Italian study report that almost 43% of recovered patients have worsened quality of life and almost 87% recovered patients have at least one persistent symptom even after two months of their recovery from the disease.
Post-recovery, some COVID-19 patients may continue to face a range of health issues, depending on the severity of the disease they explained earlier. There is limited evidence regarding long-lasting COVID-19 symptoms after the infection is gone. However, there have been reports of individuals still experiencing symptoms months after the infection, including continued loss of taste or smell, irregular heartbeats, chest pain, shortness of breath, extreme fatigue, learning difficulties, and recurring fever.

It is to be noted that not all recovered patients report long-term complications. Post COVID-19 patients who developed acute respiratory distress syndrome (ARDS) could have a higher risk of long-term health issues.
Some other complications of “long-covid” include fatigue, myalgias (muscle pain), arthralgias (joint pain), cognitive impairment, depression or anxiety, a consultation is advised if you experience any such symptoms.
Dr. Ashish Bajaj – M.B.B.S, M.D.
Post-COVID-19 patients may experience persistent respiratory symptoms, fatigue, decreased functional capacity and decreased quality of life up to 6 months after infection. People who had severe illness with COVID-19 might experience organ damage affecting the heart, kidneys, skin and brain. Inflammation and problems with the immune system can also happen.
Dr. M.G. Kartheeka, MBBS, MD

Also Read: What Causes Fingernails to Split Down the Middle: A Comprehensive Examination of the Causes
By adhering to government and local health regulatory guidance and continuing with these safety precautions, we all need to do our part to make sure the COVID-19 virus comes to an end.
It is very important for all of us to take health and safety precautions concerning COVID-19 seriously. With the reopening of businesses and public facilities, many cities are reporting an increase in the number of daily COVID-19 confirmed cases.
Next Page »« Previous Page