Mounjaro vs Zepbound: Differences, Uses, Side Effects & More
By Dr. Akash N. Shah +2 more

Get,

to manage your symptom
Get your,


4 Cr+ families
benefitted

OTP sent to 9988776655



You’ve successfully subscribed to receive
doctor-approved tips on
Whatsapp

Get ready to feel your best.

Hi There,
Download the PharmEasy App now!!


Register to Avail the Offer
Send OTPBy continuing, you agree with our Privacy Policy and Terms and Conditions

Hi There,
Sign up on PharmEasy now!!
Trusted by 4 crore+ families

OTP sent to 9988776655



You have unlocked 25% off on medicines




Code: NU25
By Dr. Akash N. Shah +2 more
Table of Contents
Mounjaro and Zepbound are two injectable medications that have gained attention for their roles in managing chronic health conditions. While both contain the same active ingredient, tirzepatide, they are FDA-approved for different uses: Mounjaro is approved for the treatment of type 2 diabetes (T2DM), whereas Zepbound is approved for chronic weight management in adults with obesity or overweight (with at least one weight-related condition)1,2.
People often compare Mounjaro and Zepbound because they share the same mechanism of action but are marketed for different health conditions. Through this article, we aim to understand the differences between Zepbound vs Mounjaro better, their effectiveness, dosing, side effects, and suitability for different health goals.

Answer a few quick questions to check eligibility.
Your response has been submitted.
Our team will contact you shortly.
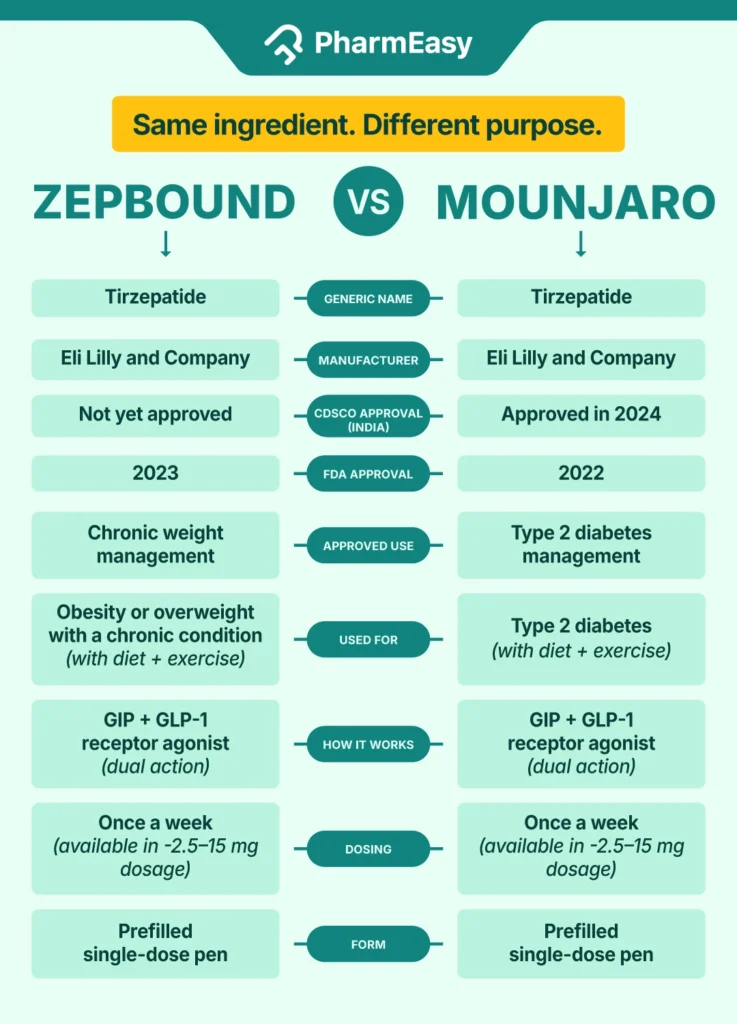
While Zepbound and Mounjaro share the same active ingredient, their approved uses and practical applications differ. Below is a side-by-side look at how they compare across key metrics3,5:
| Category | Zepbound | Mounjaro |
| Brand Name | Zepbound | Mounjaro |
| Generic Name (active ingredient) | Tirzepatide | Tirzepatide |
| Manufacturer | Eli Lilly and Company | Eli Lilly and Company |
| CDSCO Approval Year | Not approved yet in India | 2024 |
| FDA Approval Year | 2023 | 2022 |
| FDA-Approved Use | Chronic weight management | Type 2 diabetes management |
| Indications | Obesity (BMI ≥30) or overweight (BMI ≥27) with at least one weight-related condition (e.g., hypertension) | Type 2 diabetes, along with diet and exercise |
| Mechanism of Action | Dual GIP and GLP-1 receptor agonist | Dual GIP and GLP-1 receptor agonist |
| Dosing | Once-weekly injection. From 2.5 mg to 15 mg | Once-weekly injection. Doses from 2.5 mg to 15 mg |
| Starting Dose | Mounjaro 2.5mg Kwikpen (2.5 mg / 0.5 ml) | 2.5mg |
Both Zepbound and Mounjaro contain tirzepatide, a unique medication that works through dual incretin receptor agonism, targeting both GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) receptors6. This dual action sets tirzepatide apart from older treatment options that target GLP-1 alone.
GLP-1 Mechanism: GLP-1 is a hormone released in the gut after eating. Tirzepatide enhances GLP-1 activity, which6,7:
GIP Mechanism: GIP is another gut hormone that helps regulate insulin and fat metabolism. Tirzepatide mimics GIP to6,7:
The combination of GLP-1 and GIP receptor activity makes Tirzepatide highly effective in both formulations by:
Mounjaro was approved by the FDA in May 2022 for the treatment of T2DM in adults, to be used alongside diet and exercise3.
Zepbound, approved in November 2023, is indicated for chronic weight management in adults with obesity or those who are overweight and have at least one weight-related health condition (such as hypertension)4.
Regarding Mounjaro vs Zepbound dosing, both follow a once-weekly injection schedule (starting at 2.5 mg), with gradual dose increases based on individual response and tolerance3,4.
Both medications have different treatment goals despite having the same active ingredient:
Note: In the comparison of Zepbound vs Mounjaro for weight loss, both contain tirzepatide, but Zepbound is approved specifically for weight management.
While the approved indications differ, both drugs offer metabolic benefits that help address modern health challenges like obesity and diabetes.
Also Read: Liraglutide: Uses, Dosage, Side Effects & Complete Patient Guide
Looking at Zepbound vs Mounjaro side effects, both share a similar safety profile. Most side effects are related to the gastrointestinal system and tend to be mild to moderate, especially during the early weeks of treatment. However, like all medications, serious side effects are possible and should be monitored.
Here’s a side-by-side comparison of the common and serious side effects reported for both Zepbound and Mounjaro3,4:
| Side Effect Type | Zepbound | Mounjaro |
| Common Side Effects | ||
| Nausea | Yes | Yes |
| Vomiting | Yes | Yes |
| Diarrhoea | Yes | Yes |
| Constipation | Yes | Yes |
| Decreased appetite | Yes | Yes |
| Indigestion | Sometimes | Sometimes |
| Fatigue | Occasionally | Occasionally |
| Serious Side Effects (rare but possible) | ||
| Pancreatitis (inflammation of the pancreas) | Possible | Possible |
| Gallbladder problems | Possible (e.g., gallstones) | Possible (e.g., gallstones) |
| Kidney issues | Possible | Possible |
| Severe allergic reactions | Rare | Rare |
Important Safety Note: Both medications carry a boxed warning about the potential risk of thyroid C-cell tumours, although this has only been observed in animal studies. Neither Zepbound nor Mounjaro is recommended for individuals with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2)3,4.
Also Read: Wegovy Diet Plan: Complete Food Guide, Side Effect Management & Meal Ideas
Mounjaro is available in India via prescription, with costing typically starting from three thousand five hundred rupees for one dose. Zepbound has not been officially launched in India yet.
Globally, both drugs are available (primarily in the U.S. and other developed countries), with prices differing based on dosage and insurance coverage.
Note: Always consult a doctor before starting either medication. The availability, suitability, and cost of a drug can vary based on your health condition, location, and access to insurance or patient assistance programs.
Zepbound and Mounjaro contain the same active ingredient, tirzepatide, and work through the same dual hormone mechanism (GLP-1 and GIP receptor agonism). However, they are not officially interchangeable, as they are FDA-approved for different medical conditions:
Therefore, switching should not be done without medical guidance. A doctor will assess your condition, treatment goals, and possible side effects to determine the most appropriate option. Self-switching is not advised due to regulatory and therapeutic considerations.
Also Read: Wegovy (Semaglutide): How It Works, Who It’s For & What to Expect
The primary Mounjaro vs Zepbound difference lies in their approved use: Mounjaro has been approved for T2DM, while Zepbound has been approved for chronic weight management. Their dosing, side effects, and mechanisms are similar, but their FDA approvals, insurance coverage, and clinical use cases differ.
Ultimately, the right choice of medicine depends on individual health needs, including whether the focus is on blood sugar control, weight management, or both. Factors like medical history, current medications, cost, and availability also play a role in choosing the medicine. Therefore, always consult your doctor to determine which option is the safest and most effective for your specific condition. They can guide you through the risks, benefits, and practical considerations before starting treatment.
No, they should not be used together since both contain tirzepatide and would duplicate treatment and may cause potential side effects.
Yes, both contain the same active ingredient, tirzepatide, in identical forms3,4.
Not directly, substitution requires medical guidance and depends on the condition being treated.
It may increase the risk of kidney issues, especially in cases of dehydration. Therefore, close monitoring is recommended3.
People with a history of medullary thyroid cancer, MEN 2 syndrome, family history of MEN2 syndrome or serious allergic reactions to tirzepatide should avoid Zepbound4.
Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Comments

Leave your comment...
You may also like
Comments