Robust Antibody Response In Children Found With Pfizer Vaccine
By Dr. Nikita Toshi +2 more

Get,

to manage your symptom
Get your,


4 Cr+ families
benefitted

OTP sent to 9988776655



You’ve successfully subscribed to receive
doctor-approved tips on
Whatsapp

Get ready to feel your best.

Hi There,
Download the PharmEasy App now!!


Register to Avail the Offer
Send OTPBy continuing, you agree with our Privacy Policy and Terms and Conditions

Hi There,
Sign up on PharmEasy now!!
Trusted by 4 crore+ families

OTP sent to 9988776655



You have unlocked 25% off on medicines




Code: NU25
By Dr. Nikita Toshi +2 more
Table of Contents
As time passes, a larger chunk of the world population is getting vaccinated, finally signaling an end in sight for the Covid-19 pandemic. As we enter the final few months of 2021, countries are starting to think about COVID-19 vaccinations for younger demographics, especially teens. The vaccine made by Pfizer in the USA has already been approved for older children (over 12 years). Soon though, it looks like children in the 5 – 11 age bracket may be able to get the jab.
According to Pfizer, vaccination for this age group can be initiated by the time Halloween rolls around. The possibility of schools opening up soon seems very real with this news and it may only be a matter of time before we see the same trend around the world.
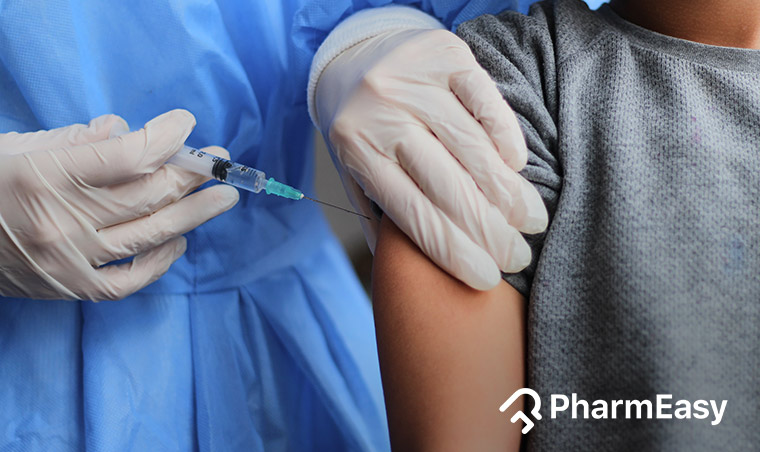
The Pfizer Covid-19 vaccine is one of the main vaccines being used in the USA to inoculate the population. As the adult population was getting their shots, attention turned to younger demographics. The Pfizer vaccine was tested and approved for emergency use on children first in Dec 2020 for those between 16-18. Later in May 2021, this was extended to those in the age range of 12 – 15. Children younger than this currently have no protection from Covid-19.
So Pfizer has been testing the vaccine at about 1/3rd the amount given to adults, in a trial covering about 2,200 children. The test found the vaccine to be safe and it generated the required antibody response. With this result, the company expects that the FDA will give Emergency Use Approval in as little as a month.
As more countries sanction vaccine trials with younger groups of children, vaccination coverage across the entire population is sure to increase very soon. Across the world, children under 15 make up 26% of the total population. The need to get them vaccinated is also important to acquire herd immunity.
With the Pfizer, Covid-19 vaccine for younger children soon to be approved, schools may finally start to end online classes. For example, in New York state there were no rules in place for student vaccination, only the staff needed to be vaccinated. This will restore stability, structure and discipline in the education system. For grownups too, this comes as welcoming news because, once this age group starts being vaccinated, parents will feel relieved and they too can resume their normal activities like going back to the workplace (which they may have been fearful of doing because they might become carriers of the disease and pass the virus on to their children).
The need for getting as many children vaccinated as possible is growing more urgent as the US is also dealing with the Delta variant. This is one of the deadliest COVID-19 variants and even if vaccinated people get infected, the symptoms are very mild and certainly not life-threatening.
The Pfizer vaccine trial on children between 5 and 11 years was conducted using 2 doses (each of 10 micrograms), with 3 weeks interval between each shot. Considering the urgent requirement at this time as well as the vaccine’s past performance with older kids and adults, this vaccine may be rolled out in as little as a few months or weeks.
Across the world, several other countries too have anticipated the need to get children vaccinated and open up schools. In India, the Zydus Cadila Covid-19 vaccine was the earliest to get emergency use approval for children as young as 12. It is also the only needle-free, plasmid DNA vaccine currently approved for people under 18, it may be used exclusively for children since it has not been used to inoculate adults yet.
In Europe, a lot of countries like France, Germany, Denmark, Sweden have begun vaccination for children 12 and over. But some of these approvals are only if the child has certain underlying conditions (lung disease, asthma. etc), others are more general.
In China, the homegrown Sinovac vaccine was approved for children 3 – 17 years. As it stands with current Covid-19 vaccines, that would be the youngest approved age. The same vaccine is being used by several countries in Africa, Asia and South America.
All through 2020 and the better part of 2021, the sheer magnitude of this pandemic seemed overwhelming. But with dedication, the scientific and the medical community are finding the means to get to the light at the end of the tunnel soon. Allowing Covid-19 vaccines for children is one of the final steps the world is taking towards being free from the pandemic. Schools were one of the first institutions to completely shut down and they might be the last to reopen. The good news is that with more trials like the one from Pfizer, there is a tangible and very real possibility that our children may finally be able to go to school in person. The world may not go back to the way it used to be but we can see the day where schools are full of children again.
Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.

Leave your comment...
Comments