All You Need to Know About India’s COVID Vaccination Drive
By Dr. Nikita Toshi +2 more

Get,

to manage your symptom
Get your,


4 Cr+ families
benefitted

OTP sent to 9988776655



You’ve successfully subscribed to receive
doctor-approved tips on
Whatsapp

Get ready to feel your best.

Hi There,
Download the PharmEasy App now!!


Register to Avail the Offer
Send OTPBy continuing, you agree with our Privacy Policy and Terms and Conditions

Hi There,
Sign up on PharmEasy now!!
Trusted by 4 crore+ families

OTP sent to 9988776655



You have unlocked 25% off on medicines




Code: NU25
By Dr. Nikita Toshi +2 more
Table of Contents
The multiple rounds of research and relentless efforts from the medical and scientific community got us our first line of defence against the novel coronavirus (COVID-19) – the vaccines.
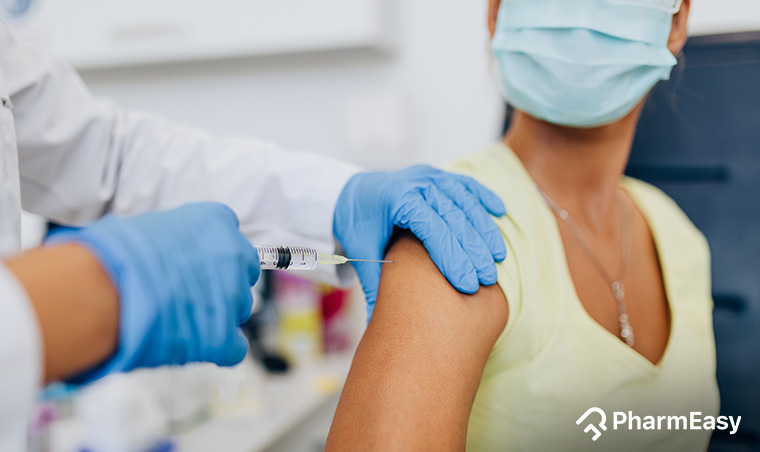
In India, we have two types of the vaccine – Covishield (manufactured by the Serum Institute of India, partnering with AstraZeneca and Oxford University, who helped develop it) and Covaxin (developed by the Indian Council of Medical Research and the National Institute of Virology and manufactured by Bharat Biotech).
These two vaccines were passed under the emergency use authorization issued by Indian regulatory bodies for immediate vaccination to curb the spread of COVID-19. The two vaccines are to be taken in two doses, with a minimum gap of 4 weeks between Covaxin and 12-16 weeks for Covishield.
Vaccines work in different ways, depending on how they have been developed. Globally, there have been 14 vaccines approved by at least one regulatory body that can be administered to the public, while many are still undergoing trials for approval.
Overall, a vaccine puts in a part of the whole of the target virus in the body, which is inactivated or weakened, thus rendered harmless when injected in the body but is potent enough to teach the immune system how to cope with it and thus produce respective antibodies. Depending on which part of the virus is used to make the vaccine, COVID-19 vaccines can be categorised as follows:
It is important to know what both vaccines have to offer. But, considering the current surge in cases and knowing that the vaccine is our only option for reducing the burden on the healthcare system, it is advisable to take whichever is available. However, people with comorbidities should consult their doctors before getting the vaccine if any ongoing medication needs to be stopped (especially for patients under immunosuppressive drugs).
Everyone above the age of 18 is eligible for vaccination, including people with existing comorbid conditions like high blood pressure, diabetes, pulmonary disorders, breathing issues, liver and kidney diseases and chronic infections which are stable and controlled by medication.
Healthcare workers (HCW) and frontline workers (FLW) should take the vaccine on a priority basis, given that they are most vulnerable to getting infected owing to the nature of their work. The elderly population should also be vaccinated on priority, owing to existing comorbidities and age.
Both vaccines are safe and have been tested through various clinical trials before being vetted by regulatory bodies. There have been reports on rare side effects of the vaccines, such as blood clots. But the benefits of the vaccines outweigh the risks by a huge margin. Moreover, patients on medications like blood-thinners can take the vaccine as it does not affect their health.
Patients with any form of anaphylactic (allergic) reaction to any food items, pharmaceutical drugs or any previous doses of vaccination (not limited to COVID-19 vaccines) are advised not to take the vaccine.
Patients suffering from COVID-19 are advised to wait for at least 3 months after recovery, before taking the vaccine. Patients who have received convalescent plasma (from another donor who has recovered in the past three months) or any other form of anti-COVID-19 antibodies or have any acute illness that may or may not require hospitalisation are also advised not to take the vaccine immediately and wait for 3 months after complete recovery. Expert medical advice is recommended in these cases.
Patients with bleeding disorders, like haemophilia, should consult their doctors for an expert opinion before taking vaccines. Similarly, patients who have been admitted to hospitals due to bleeding disorders are advised not to get vaccinated before discharge.
Children below the age of 18 are advised not to get vaccinated due to insufficient data about this age group. However, large-scale clinical trials are underway that include all age groups and this data should be available soon.
The improved immunity due to the vaccine will effectively reduce life-threatening complications caused by the novel coronavirus and reduce the number of hospitalisations. If one is adequately protected against the virus, one can also protect those around him/her, especially the elderly, those with a compromised immune system and comorbidities and the healthcare workers. This would, in turn, lead to lowering the load on the Indian healthcare system. Vaccination will also ensure fewer deaths due to COVID-19 complications. If one is getting vaccinated, one should understand the benefits of vaccination and educate others about these benefits.
The vaccinating officer asks patients to wait for half an hour inside the vaccination centre to observe any immediate adverse effects that include severe allergic reaction, increased heart rate, dizziness, swelling up of the face and throat and rashes all over the body.
Mild adverse effects include pain and swelling at the injection site, malaise, fever, body ache and headache. The vaccine officer would advise medication in case of prolonged adverse reactions. However, these reactions only last for a day or two before one is fit enough to move again.
Please remember, getting vaccinated does not mean you will not contract COVID-19 later. It means that even if one gets infected, it will not lead to severe complications/hospitalisation. Thus, continue maintaining safety protocols, wearing masks, frequent washing of hands and social distancing.
Disclaimer: The information provided here is for educational/awareness purposes only and is not intended to be a substitute for medical treatment by a healthcare professional and should not be relied upon to diagnose or treat any medical condition. The reader should consult a registered medical practitioner to determine the appropriateness of the information and before consuming any medication. PharmEasy does not provide any guarantee or warranty (express or implied) regarding the accuracy, adequacy, completeness, legality, reliability or usefulness of the information; and disclaims any liability arising thereof.
Links and product recommendations in the information provided here are advertisements of third-party products available on the website. PharmEasy does not make any representation on the accuracy or suitability of such products/services. Advertisements do not influence the editorial decisions or content. The information in this blog is subject to change without notice. The authors and administrators reserve the right to modify, add, or remove content without notification. It is your responsibility to review this disclaimer regularly for any changes.

Leave your comment...
Comments