Latest Development- Covid-19-Breathalyzer Test
By Nishkak +2 more

Download PharmEasy App




Register to Avail the Offer
Send OTPBy continuing, you agree with our Privacy Policy and Terms and Conditions
By Nishkak +2 more
Tired of the nasal and throat swab Covid-19 tests? If you are like most of us, you’ve definitely taken one of these tests and it is deeply unpleasant. Not to mention, once the swab is inserted, the nasal passage and the throat gets irritated and this tingling sensation lasts a while. Not to worry though, evolving technology has now made it possible to test for coronavirus via a breath test. Non-invasive and non-tingly, the breath test is now FDA (Food and Drug Association) approved for emergency use in selected health facilities in the US.
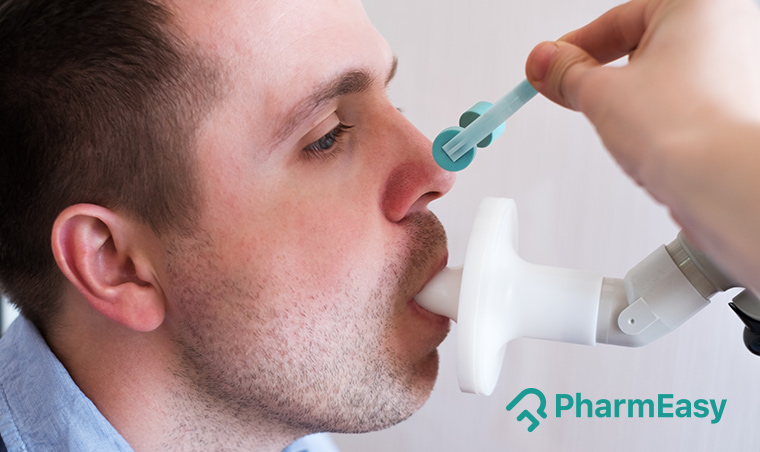
Curious to know more about this Covid-19 breathalyzer test? Read ahead to know more about the test, the usage, how to perform and the limitations.
Table of Contents
The Covid-19 breath test is a non-invasive test that checks for the presence of coronavirus in a patient by a simple breathalyzer. As more strains of the Covid-19 virus are developing, additional test methods are always welcome. Additionally, the breathalyzer test is quite useful to test patients who are allergic to the traditional nasal swab or can’t get it done due to other medical issues.
The InspectIR Covid-19 Breathalyzer test requires the patient to breathe into a tube and results are obtained in as little as three minutes. This test is slated to become the way to test the presence of coronavirus in airports, restaurants and other public gatherings.
The InspectIR Breathalyzer uses a technique called GC-MS. This technology is called gas chromatography-mass spectrometry, which separates and identifies chemical mixtures. The machine uses this GC-MS technique to detect five volatile organic compounds that are associated with the Covid-19 infection in the exhaled breath.
The breathalyzer test is ideal in large gatherings because it is fast and easy. Once the InspectIR Breathalyzer detects the presence of the Covid organic compounds, it is considered to be an unconfirmed positive test which should be confirmed via a rapid antigen test or a PCR test.
Results from the InspectIR Breathalyzer test are available in under 15 minutes. As compared to the traditional test methods, the results are obtained in no time. A PCR test gives results in two to three days (depending on the capacity of the lab), a rapid PCR test in one day and a rapid antigen test in 15 to 30 minutes.
The InspectIR Breathalyzer is small and portable, the size of a briefcase, but it needs to be administered by a trained technician, rendering the tests not ideal for home use. This test is suitable for doctor’s offices, hospitals, restaurants and other large gatherings.
Every unit is currently capable of testing about 160 samples a day and as technological advancements and R&D are currently underway, this number is expected to increase.
Also, according to InspectIR, the breath test is more effective as a screening method as compared to a temperature test. The other methods are time-consuming and costly when performed daily.
As per initial reports and tests, the Breathalyzer test is 91% efficient in identifying positive coronavirus strains and 99% efficient with negative results. Also, the scope of presenting a false negative is as low as 4.2%.
The RT PCR tests are the best at detecting positive coronavirus and even detect the smallest traces of the virus and are still considered the most reliable test.
Currently, only the Breathalyzer by InspectIR has received FDA approval, but there are other companies around the world that are engaged in research on the same. Breathonix from Singapore, GeNose C19 from Indonesia and Owlstone Medical from the UK are all in the race to get approval.
Constant development in technology and testing methods is making it possible for the world to track and tackle constant development in Covid-19 strains. As it seems like Covid-19 is here to stay, it is important to develop faster and more accurate testing methods that are non-invasive. With the breathalyzer test, InspectIR and other companies are looking to change the way we tackle and deal with the coronavirus that has taken the world by a storm. After all, change is the only constant.
Comments